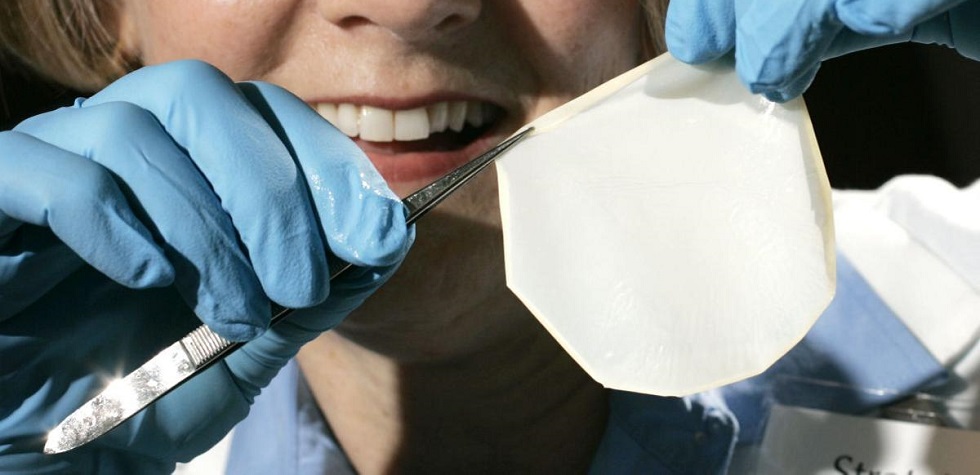
16 Jun FDA approves Madison-based skin graft technology to treat burns
The U.S. Food and Drug Administration on Tuesday approved StrataGraft, a topical treatment for severe burns made from skin tissue, providing a boost for Madison-based firm Stratatech.
The FDA approval is supported by two clinical studies involving a total of 101 adult patients. The StrataGraft treatment demonstrated effectiveness in eliminating the need for donor tissue harvest and achieved a complete wound closure in a majority of patients. There were no reports of rejection.
Steven Romano, the executive vice president and chief scientific officer at Statatech’s owner Mallinckrodt Pharmaceuticals, called the FDA’s decision a “significant milestone” in the burn care community.


Sorry, the comment form is closed at this time.